Key points:
- Nitrogen loss is based on the form of nitrogen that was applied, the use of nitrogen stabilizers, soil type, soil temperature and rainfall.
- If anhydrous was applied with a nitrification inhibitor, there is likely still sufficient nitrogen in the soil.
- Late Spring Nitrate Tests are a good way to assess if additional nitrogen is needed.
What form of Nitrogen was applied?
Anhydrous Ammonia – If anhydrous was applied in the fall or spring and a nitrification inhibitor was used most of the nitrogen is likely still in the NH4+ form and still bound to the soil therefore it is not susceptible to losses due to denitrification or leaching.
UAN – If UAN was used, 25% of the nitrogen applied is already in the nitrate form, it is likely that it has been lost if any significant rainfall has occurred. The remaining 75% of nitrogen is likely still in the NH4+ especially if a stabilizer was used and is likely still in the field.
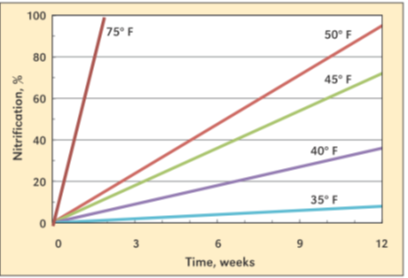
Figure 1. Nitrification percent over time based on soil temperature. (Source IPNI)
As soil temperatures reach and increase past 50 degrees the ammonium-nitrogen will be converted to nitrate-nitrogen and then it is susceptible to loss. Soil temperatures in most of Illinois and Iowa have been above 50 degrees for ~3 weeks, meaning about 20% of unstabilized NH4+ has been converted to NO3- and could be lost. Using a nitrification inhibitor with proven active ingredients and the correct rate can keep nitrogen in the ammonium form for an additional 6-8 weeks under warm soil conditions. Nitrogen loss from leaching will be higher in sandy soils. Denitrification occurs when soils are saturated, and the nitrogen is in the nitrate form. Losses of 1-2% per day can occur when soil temperatures are between 55-60 degrees. The rate of denitrification will increase as soil temperature increases.
For example:
If 100 lbs of N was applied and of soil temperatures have been around 60 degrees for 3 weeks, then roughly 20% of the nitrogen has been converted from NH4+ to NO3-. If flooded field conditions are then experienced for 5 days, about 10% of the NO3- has been lost to denitrification, equaling 2 lbs of nitrogen lost.
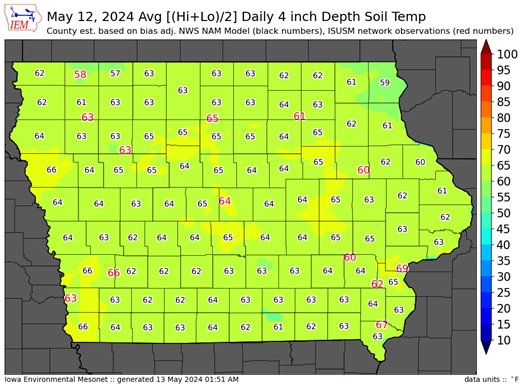
Figure 2. Soil temperature for the state of Iowa.
Iowa soil temps: https://mesonet.agron.iastate.edu/agweather/
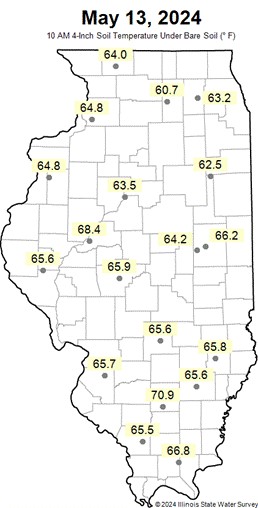
Figure 3. Soil temperature for the state of Illinois.
Illinois soil temps: https://www.isws.illinois.edu/warm/soil/
TOOLS
Late Spring Nitrate Tests
LSNT are a good tool to check the amount of nitrogen in the field. Generally, the protocol for a LSNT is to take a board with 10 holes drilled 3 inches apart and lay it down perpendicular to the anhydrous application, not to the row (or randomly if N application was broadcast). Then pull a 0–12-inch core in each one of the holes, mix and then put in bag. Send the samples to the lab and ask for an ammonium and nitrate test on each bag. Ideally, there is 25 ppm of nitrate-nitrogen in the top 12 inches and some nitrogen could be remaining in ammonium form. The second 12 inches is to show if any nitrate has moved down there. This interpretation is based on 6-12-inch-tall corn.
Adapt - N®
There are also tools such as Adapt-N® that can help model nitrogen on a broader scale by considering the weather, crop rotation, previous nitrogen applications, yield goals, and soil types. This tool will model the nitrogen environment in different areas of the field and provide an estimate of what you may still need to apply.
Sidedress application
If UAN was used as the primary source of nitrogen, it is likely that 25% has already been lost this spring and should be replaced with a sidedress application. If the LSNT or modeling tool indicate the field is low on nitrogen a sidedress application may be necessary to maximize yield. Additional considerations are the timing of the anhydrous application in the fall and if a nitrification inhibitor was used. If anhydrous was applied when soil temperatures were still warm last fall, some of the nitrogen may have already converted from NH4+ to NO3- and be susceptible to loss. Similarly, if a nitrification inhibitor was not used, it is likely that the nitrogen that has been converted from NH4+ to NO3- is lost.