- Organic matter contains a lot of bound organic nitrogen only available to the crop thru OM mineralization
- Mineralization is driven by temperature, soil moisture, soil aeration and dependent on optimum soil pH (6.0-7.0)
- Forms of nitrogen act differently in the soil, nitrate moves freely about with soil moisture, while ammonium attaches to CEC. Nitrate moves thru mass flow to the plant and is dependent on plant transpiration, while ammonium uptake requires direct root intercept
- The plant has no preference whether the nitrogen taking up is nitrate or ammonium in form. However nitrate is much more readily available due to it moving about in the soil solution thru mass flow.
Large or multiple rain events always create questions and concerns about applied nitrogen. Here are some of the basic questions and corresponding facts to consider.
How much N can be found in the soil? If an Acre Furrow Slice (AFS – 6 2/3 inches deep) weighs 2,000,000 pounds and has 3% Organic Matter (OM), there would be approximately 3,000 pounds of N per acre (60,000 pounds OM x 5% N). Refer to the table at the end of the article. This table can help estimate pounds of N per acre (in the AFS) based upon soil O.M.
Why apply N for corn with the abundance of N found in the soil? Over 95% of the N found in soil is bound organically. Although an organic form of N is stable, it is also not available for plant uptake. The N captured within organic crop residue/organic matter must first be released through mineralization to an inorganic form that the plant can utilize (nitrate or ammonium-N), this process is sometimes called ammonification. Mineralization is driven by microbial activity, or the soil’s biology. Factors that influence the speed of mineralization include soil organic matter, temperature, moisture, aeration, and pH. Mineralization can account for relatively large additions of nitrogen to the soil environment in some years. In 2016, mineralization was believed responsible for relatively large amounts of N detected being detected in spring nitrogen testing at N-watch sites and in University of Illinois nitrogen trials. Mineralization is one of the more difficult parts of the nitrogen cycle to computer model due to the dynamics involved.
Is the form of N important in the soil? There are two forms of N available to a plant, nitrate-N and ammonium-N, both act differently in the soil. Ammonium-N is reduced (without oxygen) and has a positive charge when in soil solution. Since it has no oxygen attached, it cannot be lost through denitrification (turning into a gas). Since it has a positive charge, its movement is limited in the soil due to its attraction to the soil’s negatively charged sites; this relationship is foundational to the Cation Exchange Capacity or CEC. Nitrate-N is an oxidized form of plant-available N in the soil, and can be lost under anaerobic (without air) soil conditions caused by flooding or saturation over time. Furthermore nitrate-N has a negative charge in soil solution and has the freedom to move wherever soil water moves since it is not attracted to the soil’s Cation Exchange Capacity, (soil is also negative, & negative charges repel similar to like poles of a magnet).
Is the form of N important to the plant? The primary form taken up by the plant is nitrate-N, but the quantity of N is of primary importance to the plant. The plant needs a large volume of N and the only way to get enough into the plant to meet plant demand is through mass flow in soil water. As a vector, mass flow, is created which via plant transpiration as the plant naturally cools itself by losing moisture thru leaf stoma. Nitrate-N moves into the plant with water pulled from the soil through the root system as water evaporates from the leaf’s surface. If all the plant’s N demand was supplied with ammonium-N, a form of N that does not move freely with soil water, the plant would end up “N deficient”.
Nitrogen management strategies should focus on providing “plant-available N,” from both nitrate and ammonium-N, to the plant when needed in order to maximize N utilization. The microbial population of the soil will determine the plant-available form. Nitrification (microbial conversion of ammonium to nitrate-N) occurs within two weeks in warm, moist soils. Low concentrations of ammonium-N are quite common all summer long due to this process. There are likely many other yield-limiting factors that will need to be satisfied before ammonium nutrition becomes the primary limiting factor. According to Liebig’s Law of the Minimum, it is the lowest stave in the barrel (most limiting nutrient) that will most limit harvest yield.
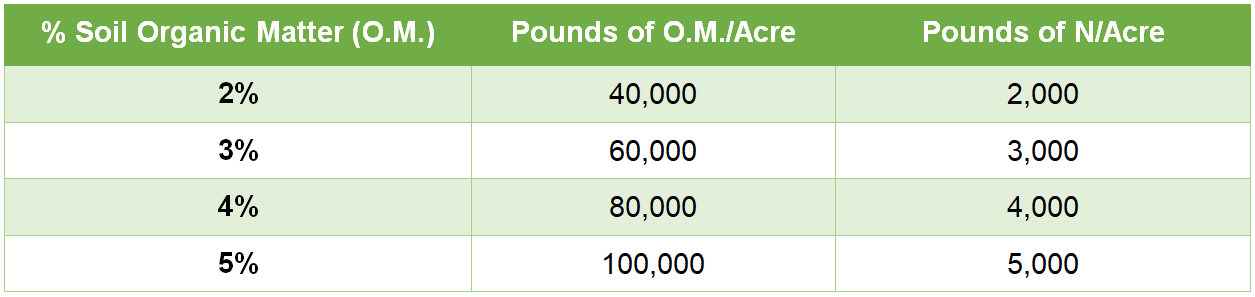
Photo: Urea Topdressing on Corn (Prairieland FS)